Summary of Minor Research Project Report on
" Specific Heat and Thermal Expansion of Multiferroic CMR Manganites"
Under Grant FR. No. MS-121/102054/12-13/CRO
Submitted to
University Grants Commission, Central Regional Office, Bhopal.
2014
According to the original definition put forward by Schmid, multiferroic materials are materials that combine two or more of the primary forms of ferroic order, i.e. ferroelasticity, ferroelectricity, ferromagnetism, and ferrotoroidicity. The prime examples of manganites having multiferroic properties are Pb(B1/2B'1/2)O3 (B=Fe,Mn,Ni,Co; B'=Nb,W,Ta), BiMnO3, Bi(Fe0.5Cr0.5)O3, (Y,Yb)MnO3, HoMnO3, InMnO3, Y(Ho)MnO3,Pr1-xCaxMnO3, RMnO3 (R=Tb,Dy)[2]. The focus of current investigation will be on revealing the specific heat and thermal expansion of most of these manganites.
Motivated from these facts, the author thought of making a systematic study of manganites using an interatomic potential with minimum number of adjustable parameters to predict the cohesive, elastic and thermal properties of doped manganites. The cations of varying sizes and valence (monovalent, divalent cations) were doped at the A-site of the parent AMnO3 perovskite manganites and their effect on the lattice specific heat of manganites was studied systematically.
The Rigid Ion Model (RIM) was formulated to include long-range (LR) coulomb interaction, van der Waals (vdW) interaction and short-range (SR) Hafmeister-Flygare (HF) overlap repulsion operative upto second nearest neighbour. The potential describing the interatomic interactions within the RIM formulation is expressed as:
![]() where the first term is the LR Coulomb attraction potential expressed as:
where the first term is the LR Coulomb attraction potential expressed as:
![]() with rkk΄ as the separation between the ions k and k´. Zk (Zk´) are the ionic charges of ions k(k´). e is the electronic charge. The overlap repulsive energy
with rkk΄ as the separation between the ions k and k´. Zk (Zk´) are the ionic charges of ions k(k´). e is the electronic charge. The overlap repulsive energy![]() , according to Hafemeister-Flygare type interaction extended upto the second neighbour ions, is expressed as:
, according to Hafemeister-Flygare type interaction extended upto the second neighbour ions, is expressed as:
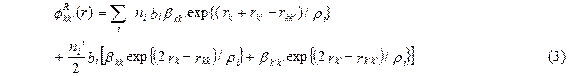 Here, k (k') denotes the positive (negative) ions and the sum is taken over all the ions.
Here, k (k') denotes the positive (negative) ions and the sum is taken over all the ions.
Zk (Zk´)and N k (Nk´) are the valence and the number of electrons in the outermost orbits and rkk΄ and rkk (= rk΄k΄) are the first and second neighbour separations, respectively. rk (rk΄) are the ionic radii of k (k′) ions. n (n′) is the number of nearest (next nearest neighbour) ions. bi and ri are the hardness and range parameters for the ith cation-anion pair, respectively. The third term is van der Waal’s interaction due to dipole-dipole and dipole-quadrupole interactions
The model parameters, hardness (b) and range parameter (r), are determined from the equilibrium condition:
![]() and the bulk modulus:
and the bulk modulus:
 where, K is the crystal structure constant dependent on the crystal structure and ro is the equilibrium nearest neighbor distance.
where, K is the crystal structure constant dependent on the crystal structure and ro is the equilibrium nearest neighbor distance.
The interatomic potential (eqn.1 to 5) and other eqns. are applied to investigate the various static properties like cohesive energy (![]() ), Compressibility (β), Molecular force constant (f), Reststrahlen frequency (n0), Debye temperature (qD), Specific Heat (CV), Thermal expansion (a) and Grüneisen parameter (g) of some multiferroic manganites within the harmonic approximation of vibrations of the atoms. The results reported in the present work had been mostly published in reputed international journals and presented in the proceedings of national/ international conferences / symposia. The entire work presented in this thesis was carried out at sri sathya Sai College for Women, Bhopal by the author.
), Compressibility (β), Molecular force constant (f), Reststrahlen frequency (n0), Debye temperature (qD), Specific Heat (CV), Thermal expansion (a) and Grüneisen parameter (g) of some multiferroic manganites within the harmonic approximation of vibrations of the atoms. The results reported in the present work had been mostly published in reputed international journals and presented in the proceedings of national/ international conferences / symposia. The entire work presented in this thesis was carried out at sri sathya Sai College for Women, Bhopal by the author.
By Dr. Archana Srivastava
Asstt. Prof., Department of Physics,
Sri Sathya Sai College for Women, Bhopal 462024